Chapter 6
6 Membrane Stains
Dyes may be used to distinguish different structures on the vitreoretinal interface, including the posterior hyaloid face, epiretinal membranes (ERM) and the internal limiting membrane (ILM). Although surgery at the vitreoretinal interface may be performed without dyes by the experienced surgeon, the use of dyes is especially helpful for beginner surgeons and may be required for achieving good surgical results and safety.
The ideal dyes used for vitreo-retinal interface surgery should have the following properties: high biocompatibility, water solubility, provide good visualization (rapid and specific tissue absorption and contrast), non-toxicity, physiologic degradability and practicality, such as dyes with dual staining property. The following dyes are commonly used for vitreo-retinal interface surgery and can be injected with a soft-tip cannula:
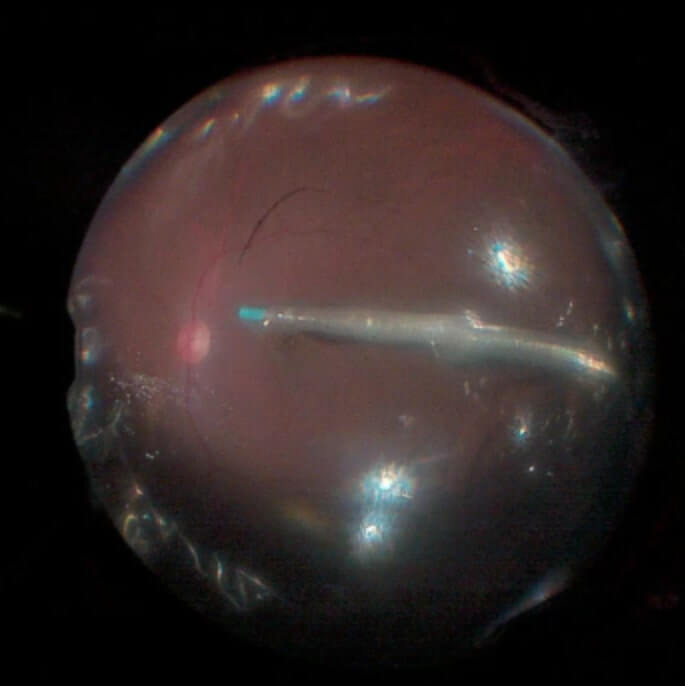
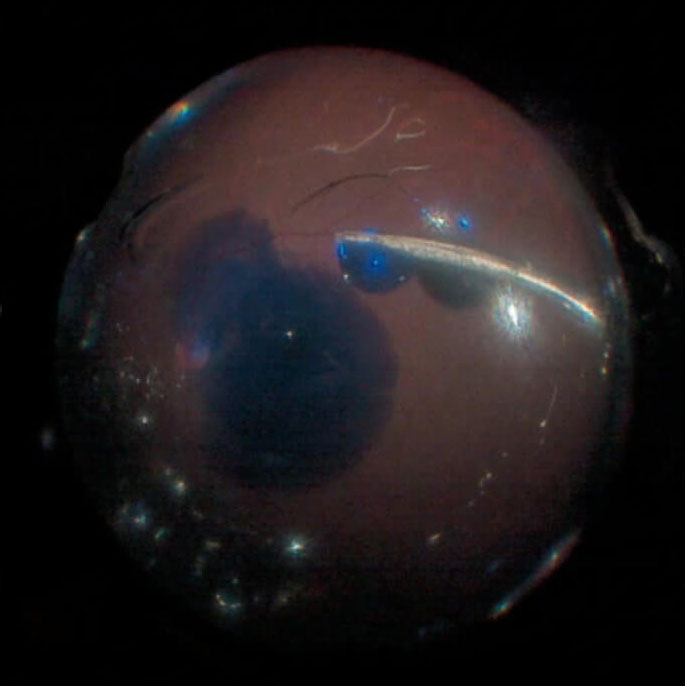
Triamcinolone acetonide is best for staining vitreous and the posterior hyaloid face (Figure 6.1) but can also be used to visualize and peel of the ILM.[1] It can also reduce intraocular inflammation including macular edema. Postoperative intraocular pressure should be monitored carefully for a steroid response, especially in patients with glaucoma or with existing high intraocular pressure. Both 10 mg/ml and 40 mg/ml suspensions can be used, but it is often helpful to dilute the triamcinolone with BSS® since dense triamcinolone can in itself impede the view. Some commercial preparations of TA are approved for intraocular use.[1]
Couch SM, Bakri SJ. Use of triamcinolone during vitrectomy surgery to visualize membranes and vitreous. Clin Ophthalmol. 2008;2(4):891-6.
Trypan Blue (e.g. MembraneBlue™, DORC) is a useful stain for visualizing ERM and PVR membranes but not the ILM. It is US Food and Drug Administration (FDA) approved. It is considered less toxic than indocyanine green but has rarely been reported to cause RPE atrophy[2] and CME.[3] Caution should be taken when using it in phakic patients as it may stain the posterior capsule and inhibit the view. This is not usually a problem with newer formulations that sink, but if there is still concern TB can be injected under air then removed prior to going back to a full fluid fill.
Jain S, Kishore K, Sharma YR. Progressive atrophy of retinal pigment epithelium after trypan-blue-assisted ILM peeling for macular hole surgery. Indian J Ophthalmol. 2013;61(5):235-7.
Gouws P, Merriman M, Goethals S, Simcock PR, Greenwood RJ, Wright G. Cystoid macular oedema with trypan blue use. Br J Ophthalmol. 2004;88(10):1348-9.
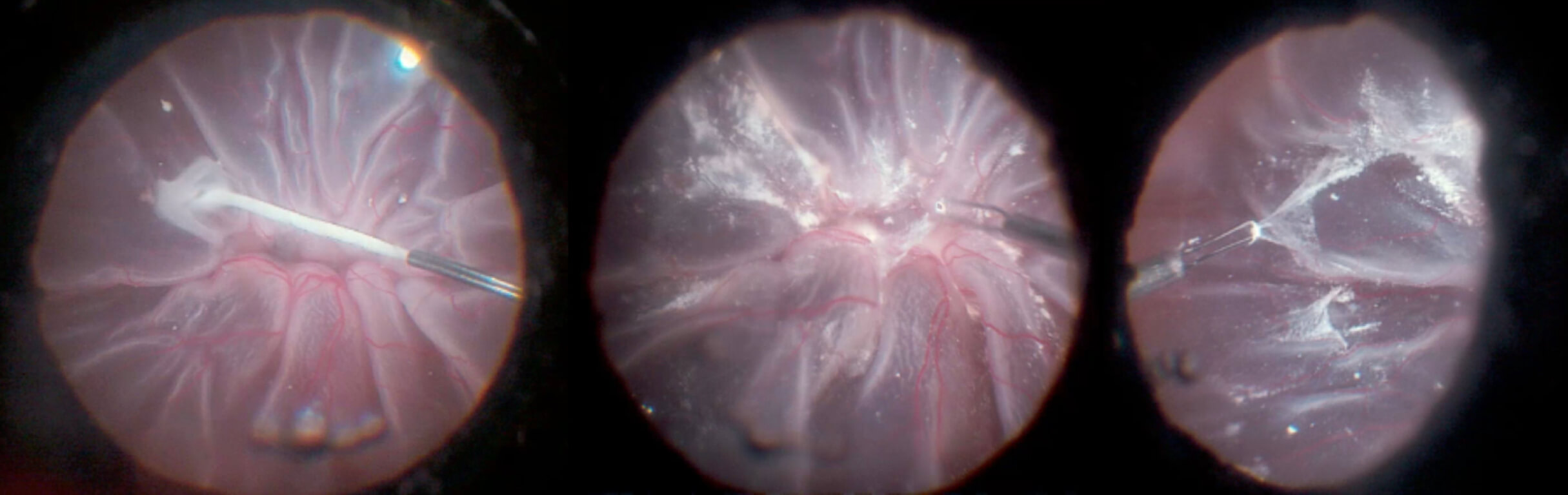
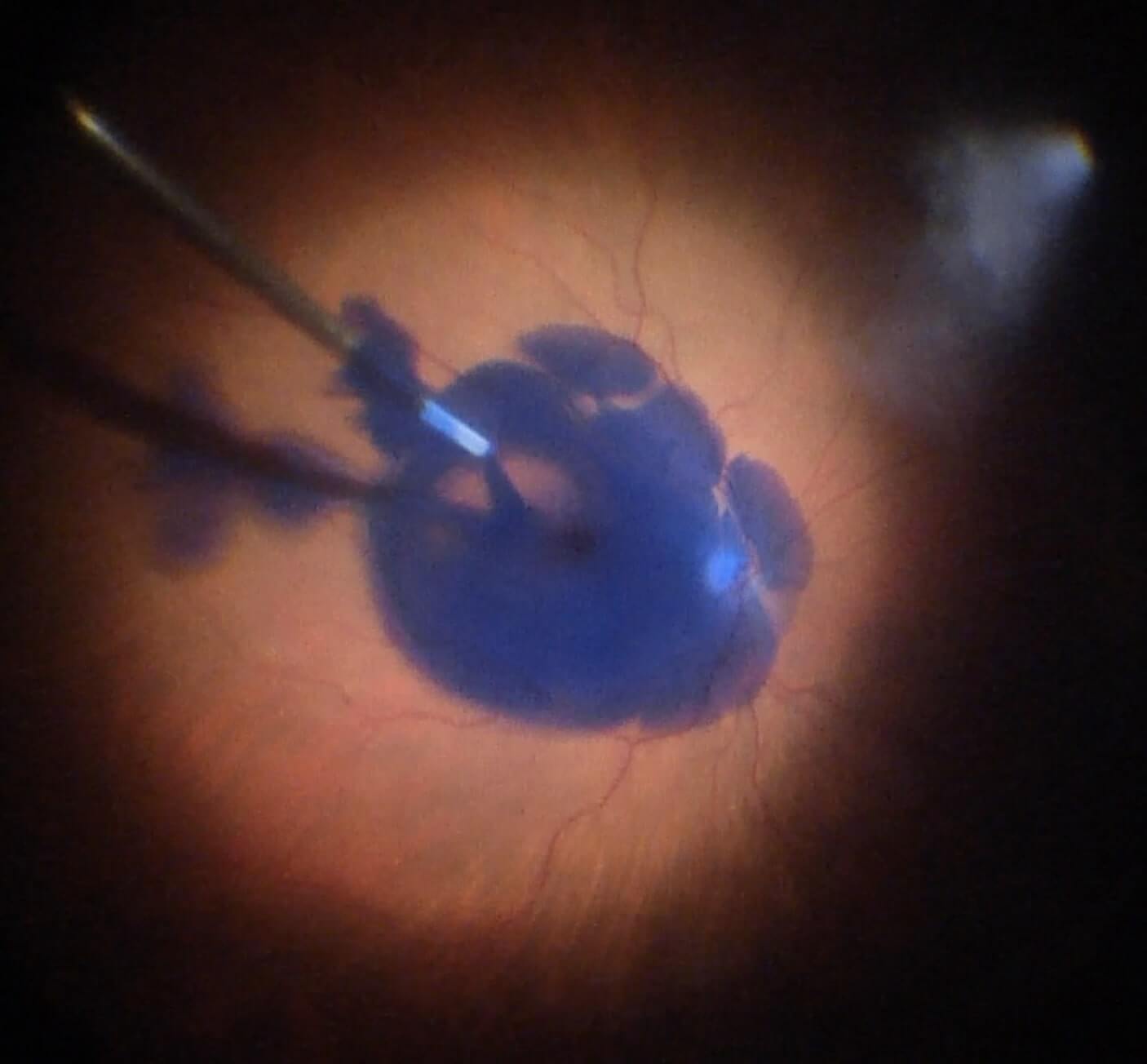
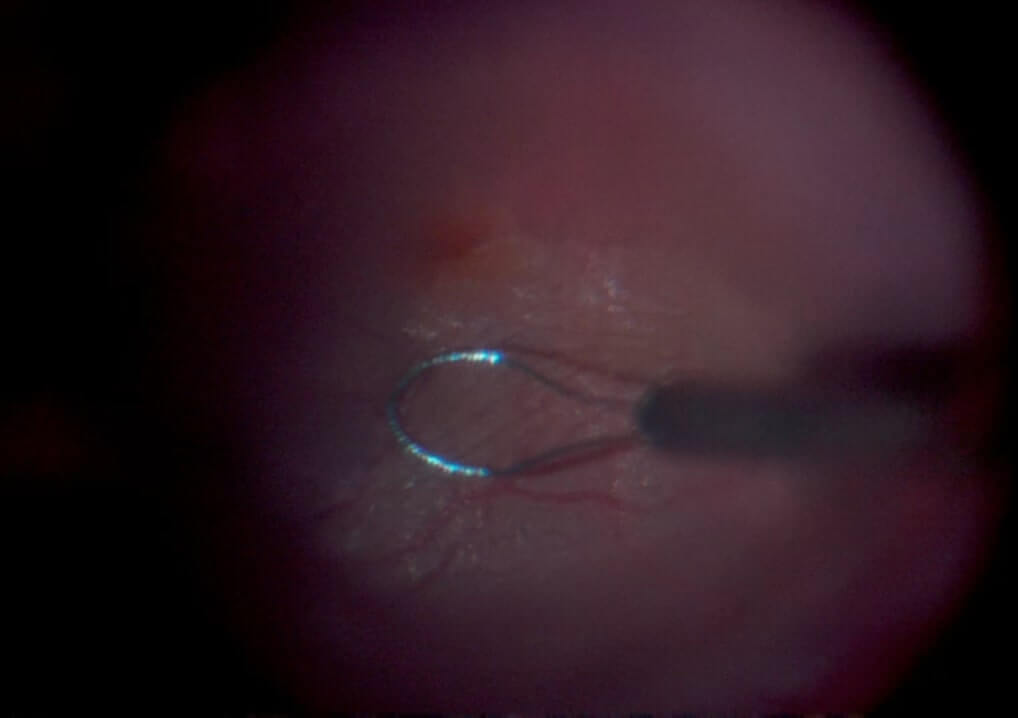
BBG (e.g. ILM-Blue™, DORC) preferentially stains the ILM (Figure 6.2) and may cause less toxicity than both trypan blue and indocyanine green (ICG) dye . BBG is translucent, which provides better visibility when the eye is dye-filled (Video 6.1).
The combination of BBG 0.025% and TB 0.15% (e.g. Membrane Blue-Dual™, DORC) was developed to provide dual stain properties of both ERM and ILM (Video 6.2).
The combination of BBG 0.025% and TB 0.15% (e.g. Membrane Blue-Dual™, DORC) was developed to provide dual stain properties of both ERM and ILM (Video 6.2).
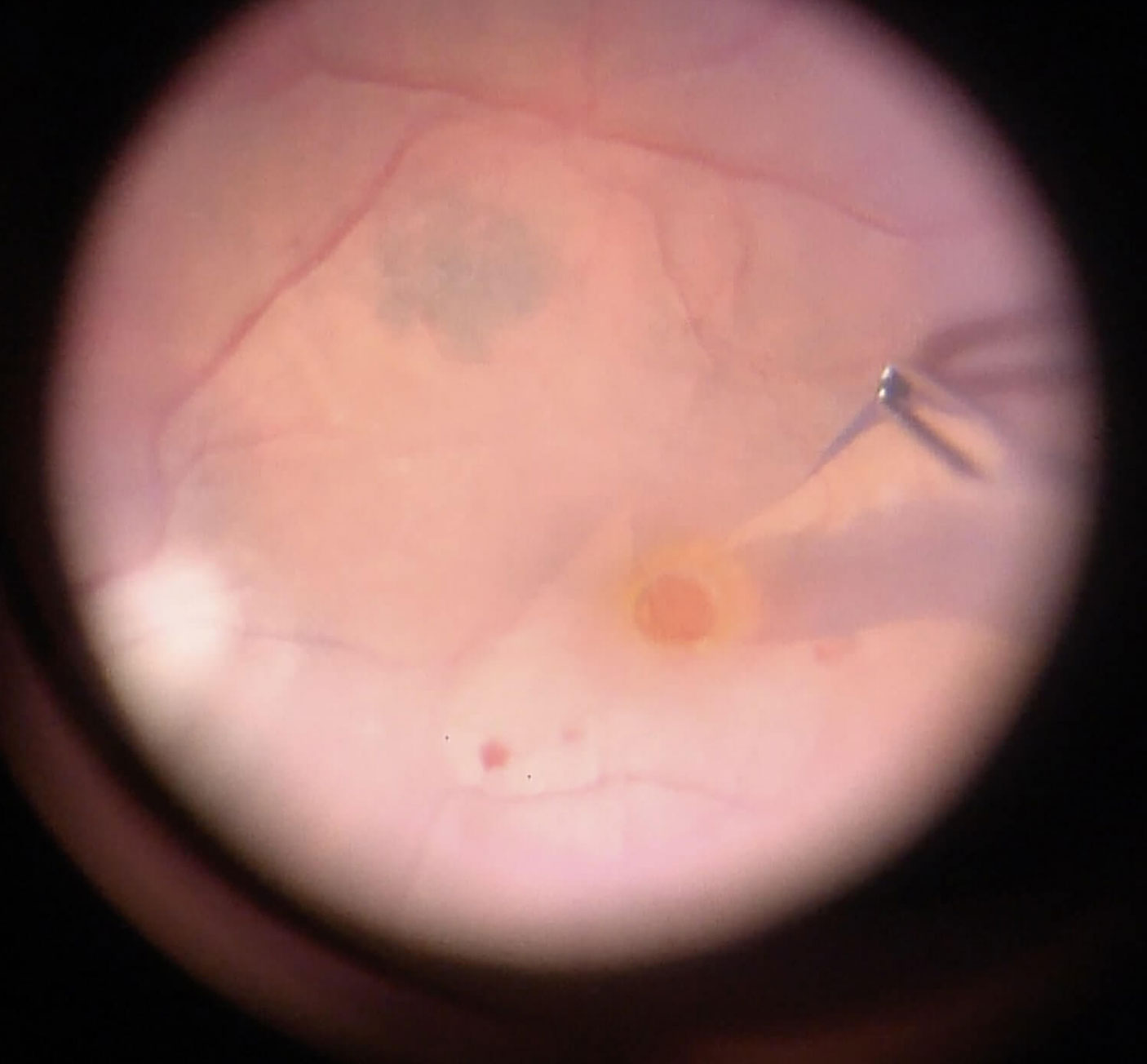
Figure 6.2 Brilliant Blue G (BBG) Used To Stain The ILM For Macular Hole Repair
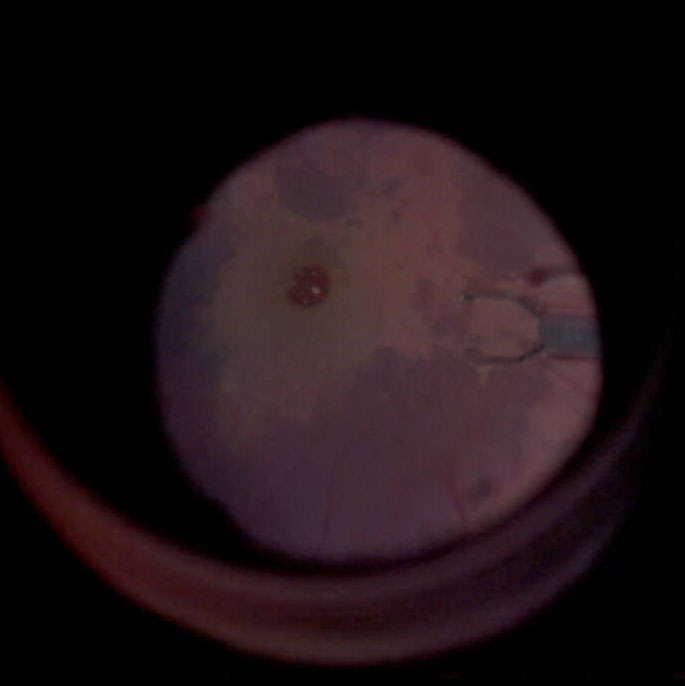
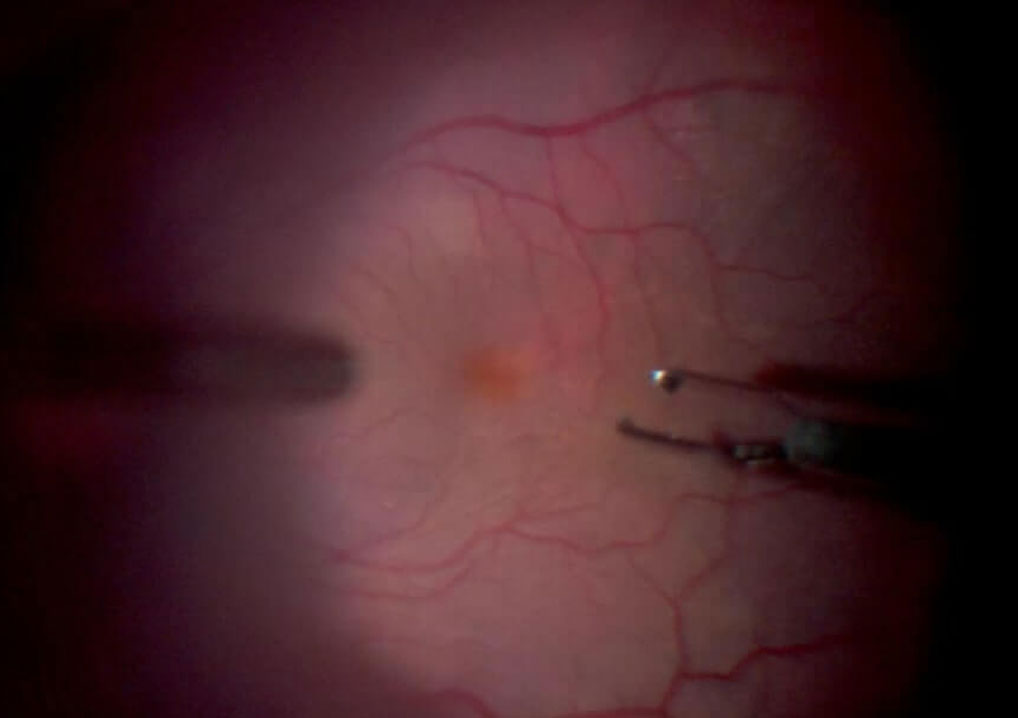
C. The BBG is removed after allowing a staining time of approximately 30 seconds
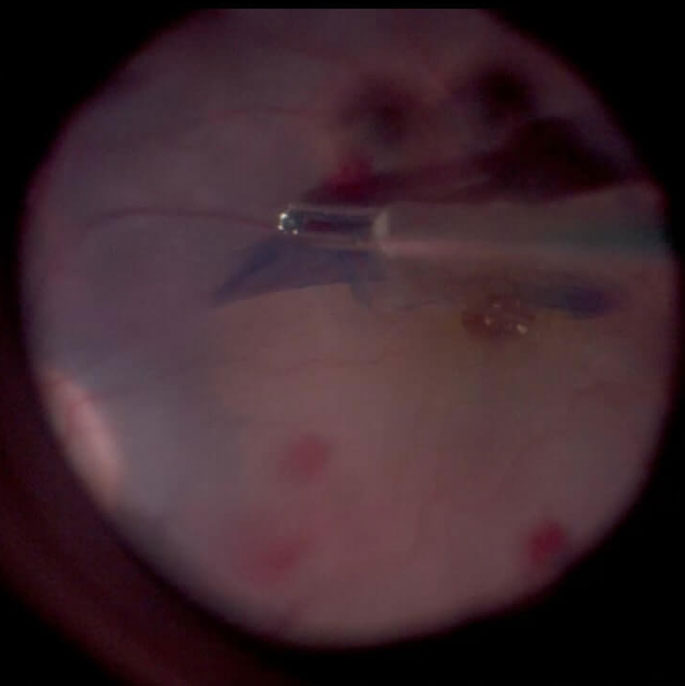
D. ILM peeling. Please note the contrast between the stained ILM grasped with the forceps (blue) and the naked retinal surface below (tan)
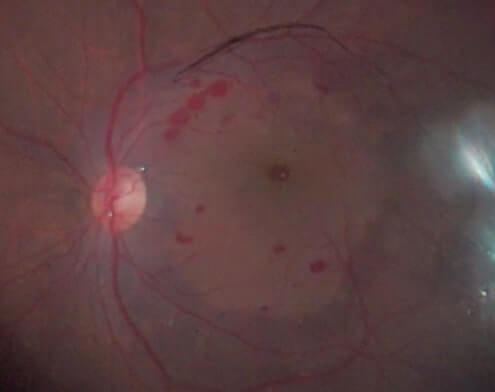
E. After ILM peeling, the retinal surface without ILM appears without blue color, in contrast with the surrounding area with blue color due to intact ILM with BBG staining.
Note
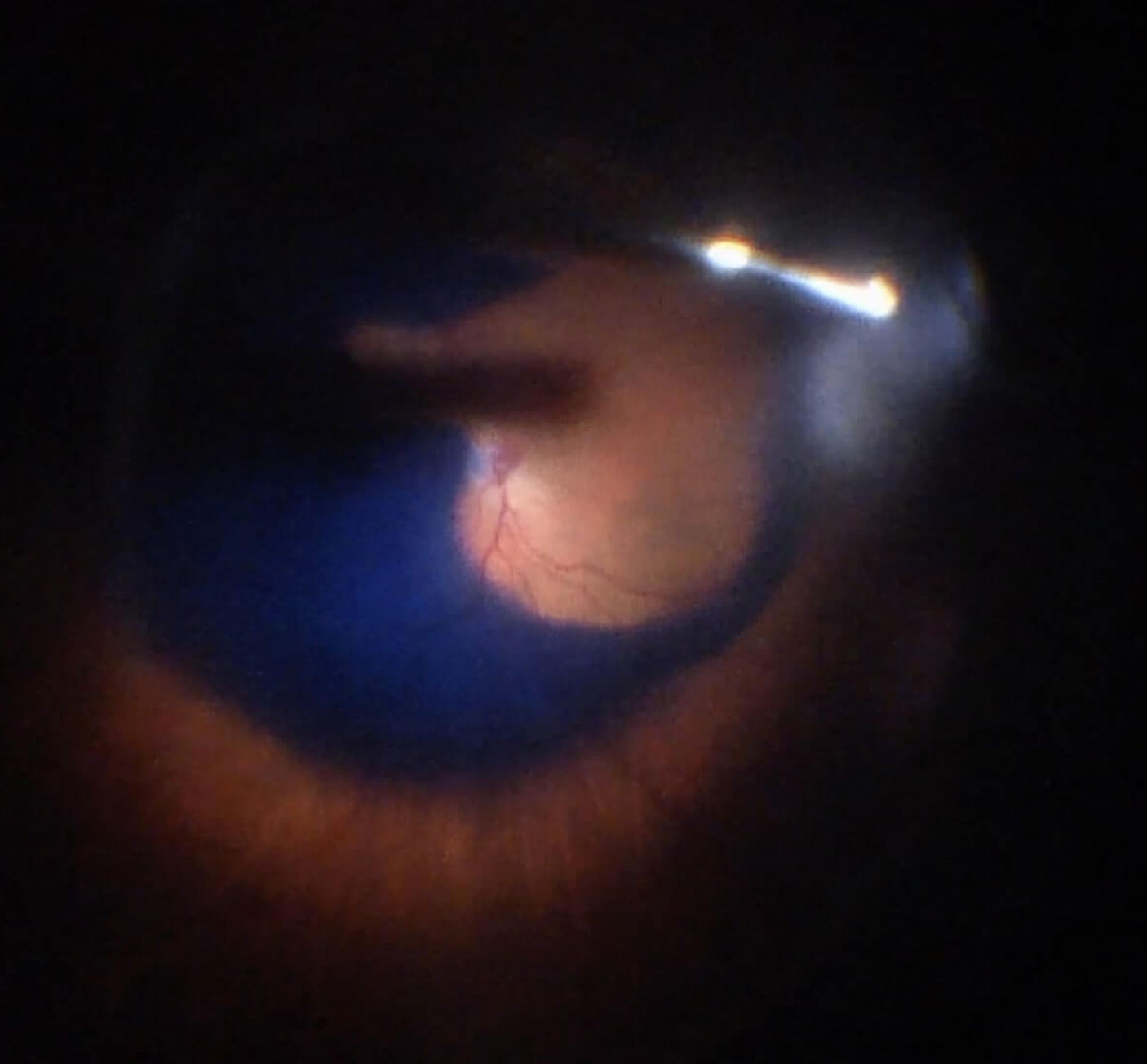
Brilliant Blue G stains may be blocked by ERMs. This leaves a paler area (“negative stain”) where the ERM is, compared with the area of the ILM without ERM which stains positively (Figure 6.4)
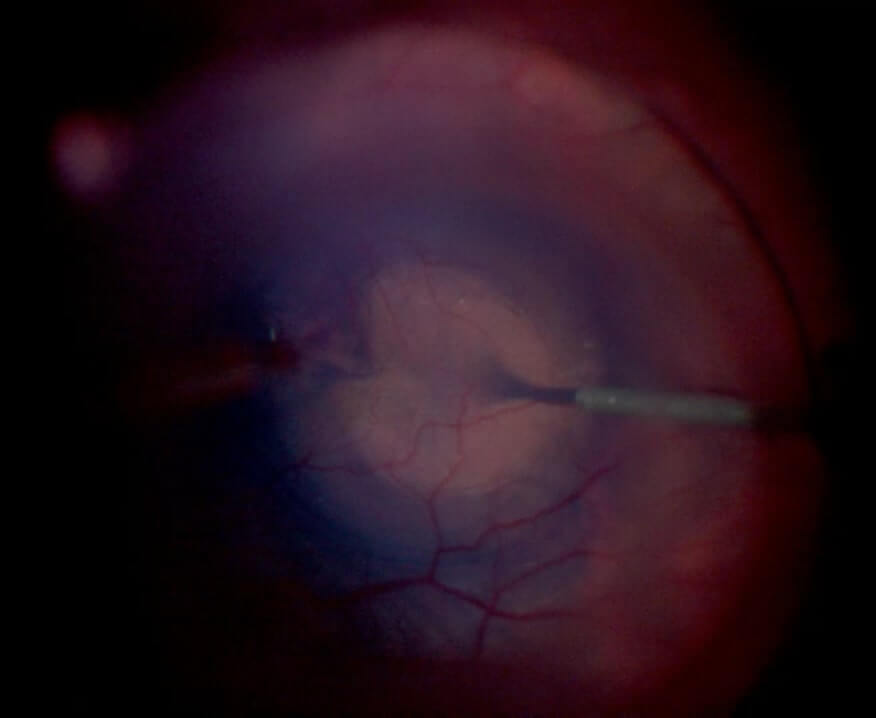
C. Repeat BBG injection
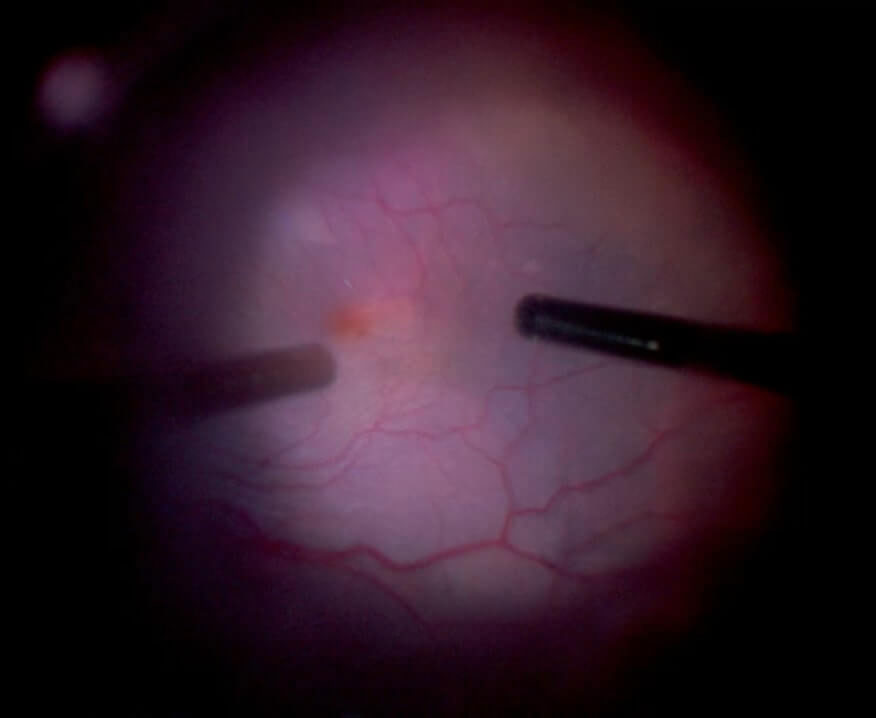
D. The ILM now stains positively
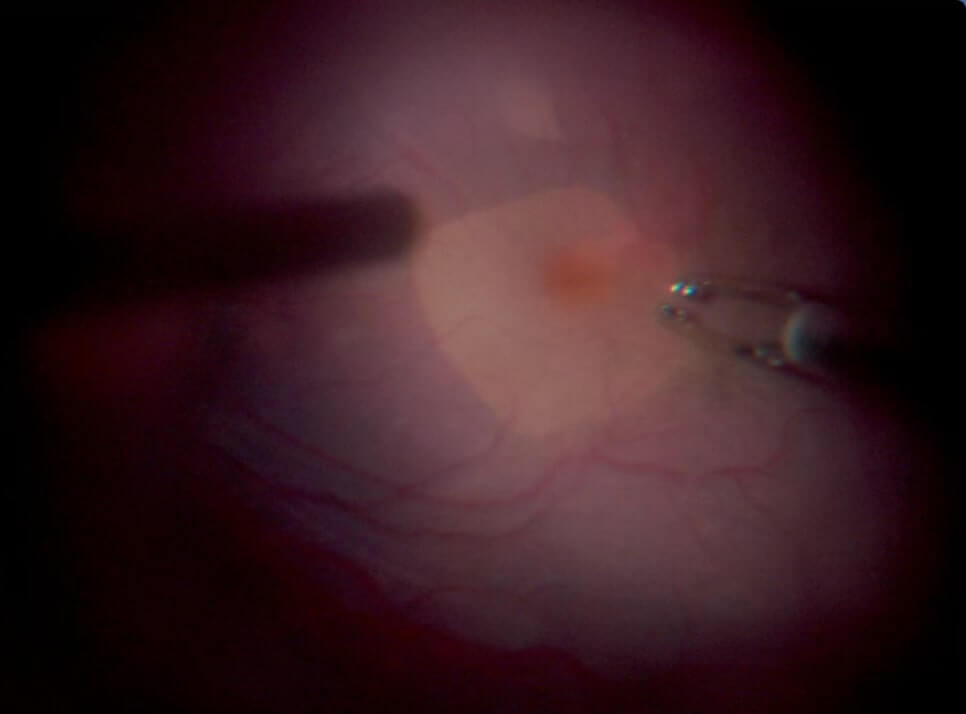
E. Area of pale-colored retina after ILM peeling.
All rights reserved. No part of this publication which includes all images and diagrams may be reproduced, distributed, or transmitted in any form or by any means, including photocopying, recording, or other electronic or mechanical methods, without the prior written permission of the authors, except in the case of brief quotations embodied in critical reviews and certain other noncommercial uses permitted by copyright law.
Westmead Eye Manual
This invaluable open-source textbook for eye care professionals summarises the steps ophthalmologists need to perform when examining a patient.