2.6 Robotics
In vitreoretinal surgery, the manipulation of delicate tissues and structures requires a very precise positioning of instruments relative to their targets, often within about 10 microns.[1,2,3,4] This degree of precision is well below the 100 micron amplitude of the natural tremor present in a human hand.[5] Furthermore this surgery often requires certain static maneuvers, such as holding a cannula in a small structure (e.g. retinal vessel, sub-retinal space) without movement. Hence, a micro-manipulator or a robotic assistant can be of benefit to carry out high precision maneuvers with more ease and safety.
Robotic surgery took place as early as 1988 in the setting of stereotactic neurosurgery.[6] The first reported use of a micro-manipulator to facilitate retinal surgery occurred 5 years earlier in 1983.[7] The ongoing miniaturization of ophthalmic surgical instruments displaced the need for the micro-manipulator a few years later, and as a result, the drive to develop robotics in ophthalmology paused for several years.
The recent adoption of high precision imaging (e.g. intra-operative OCT, iOCT) in surgery and the need to carry out delicate site-specific procedures in the subretinal space (e.g. gene therapy) has reinitiated the interest for high precision robotics in our discipline.
Riviere CN, Ang WT, Khosla PK. Toward Active Tremor Canceling in Handheld Microsurgical Instruments. IEEE Trans Robot Autom. 2003. doi:10.1109/TRA.2003.817506
de Smet MD, de Jonge N, Iannetta D, Faridpooya K, van Oosterhout E, Naus G, Meenink TCM, Mura M, Beelen MJ. Human/robotic interaction: vision limits performance in simulated vitreoretinal surgery. Acta Ophthalmol. 2019 Nov;97(7):672-678. doi: 10.1111/aos.14003. Epub 2018 Dec 27. PMID: 30588753.
de Smet MD, Naus GJL, Faridpooya K, Mura M. Robotic-assisted surgery in ophthalmology. Curr Opin Ophthalmol. 2018 May;29(3):248-253. doi: 10.1097/ICU.0000000000000476. PMID: 29553953.
Molaei A, Abedloo E, de Smet MD, Safi S, Khorshidifar M, Ahmadieh H, Khosravi MA, Daftarian N. Toward the Art of Robotic-assisted Vitreoretinal Surgery. J Ophthalmic Vis Res. 2017 Apr-Jun;12(2):212-218. doi: 10.4103/jovr.jovr_63_17. PMID: 28540014; PMCID: PMC5423376.
Singhy SPN, Riviere CN. Physiological tremor amplitude during retinal microsurgery. In: Proceedings of the IEEE Annual Northeast Bioengineering Conference, NEBEC. 2002. doi:10.1109/NEBC.2002.999520
Kwoh YS, Hou J, Jonckheere EA, Hayati S. A robot with improved absolute positioning accuracy for CT guided stereotactic brain surgery. IEEE Trans Biomed Eng. 1988 Feb;35(2):153-60. doi: 10.1109/10.1354. PMID: 3280462.
Spitznas M. Motorized teleguided stereotactic micromanipulator for vitreous microsurgery. Arch Ophthalmol. 1983 Apr;101(4):623-30. doi: 10.1001/archopht.1983.01040010623020. PMID: 6838423.
Ophthalmic robots now in existence or in development can be classified into three main categories:[3,8,9]
de Smet MD, Naus GJL, Faridpooya K, Mura M. Robotic-assisted surgery in ophthalmology. Curr Opin Ophthalmol. 2018 May;29(3):248-253. doi: 10.1097/ICU.0000000000000476. PMID: 29553953.
Kummer M, Abbott J, Kratochvil B, Borer R, Sengul A, Nelson B. An electromagnetic system for 5‑DOF wireless micromanipulation. IEEE Trans Robot Autom. 2010;(26):1006-1017.
Hubschman JP, Bourges JL, Choi W, Mozayan A, Tsirbas A, Kim CJ, Schwartz SD. 'The Microhand': a new concept of micro-forceps for ocular robotic surgery. Eye (Lond). 2010 Feb;24(2):364-7. doi: 10.1038/eye.2009.47. Epub 2009 Mar 20. PMID: 19300461.
- Assistive hand-held instruments
- Co manipulation platforms
- Tele manipulation systems
All major features are summarized in Table 1
Feature
Assistive Hand-held
Co-manipulator
Tele-manipulator
Tremor Filtering
AH
+
CM
+
TM
+
Motion Scaling
AH
-
CM
+
TM
+
Remote Center of Motion (RCM)
AH
-
CM
+
TM
+
Eye Stability
AH
-
CM
+
TM
+
Motion Profiles and Task Automation
AH
-
CM
-
TM
+
Improved Static Tasks
(e.g. Drug Delivery)
AH
+
CM
+
TM
+
Intuitive Control
AH
+
CM
-
TM
±
Remote Control Option
AH
-
CM
-
TM
+
Rapid Exit
AH
+
CM
-
TM
+
Table 1: Key Features and Properties of Ophthalmic Robotic Systems per Configuration
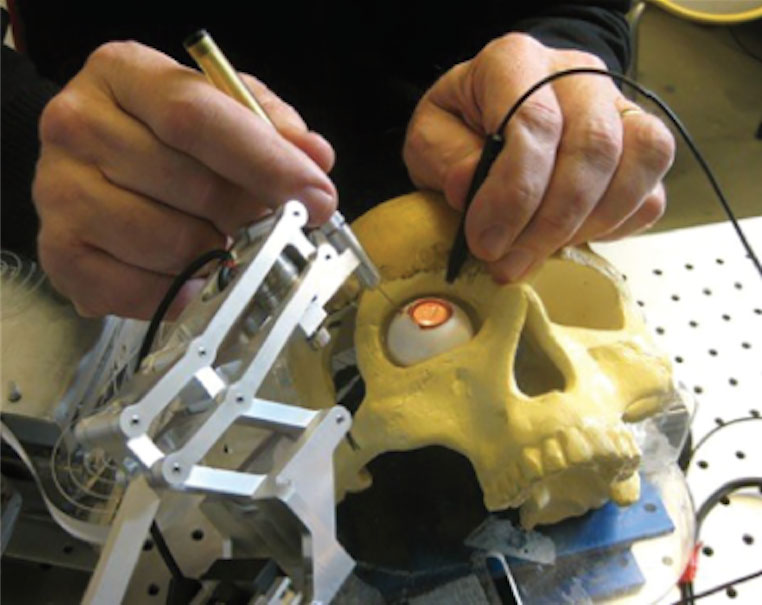
Assistive hand‑held instruments are special tools that aim to compensate for tremor and unintentional movements in the surgeon’s hand.[10] The surgical instrument is mounted on a hand‑held compensating device that stabilizes and cancels out unwanted fine movements (Figure 2.6.1).
Becker BC, Yang S, Maclachlan RA, Riviere CN. Towards Vision-Based Control of a Handheld Micromanipulator for Retinal Cannulation in an Eyeball Phantom. Proc IEEE RAS EMBS Int Conf Biomed Robot Biomechatron. 2012 Dec 31;2012:44-49. doi: 10.1109/BioRob.2012.6290813. PMID: 24649479; PMCID: PMC3955894.
Figure 2.6.1
Micron, a hand-held robotic instrument, developed at Carnegie Mellon University (CMU) is designed to remove unintentional hand movement and compensating for hand tremor.
(from Molaei A, Abedloo E, De Smet M, et al. Toward the art of robotic-assisted vitreoretinal surgery. J Ophthalmic Vis Res. 2017.)
A co-manipulation system uses a mechanism designed to facilitate specific surgical tasks. It can, for example, stably hold an instrument (catheter) in a vessel, for a prolonged period of time. During a surgical maneuver, the manipulator dampens movements, thereby limiting tremor. It can also maintain a stable position independent of the surgeon’s grip, further extending the physiologic reach of a surgeon.[11,12,13] Co-manipulation avoids the need for a separate joystick, or the need for a sophisticated computerized control mechanism, reducing the cost of the system . However, it comes at the cost of limited flexibility, dampening and hindering the movement of the surgeon’s intraocular movements (Figure 2.6.2).
de Smet MD, Stassen JM, Meenink TC, Janssens T, Vanheukelom V, Naus GJ, Beelen MJ, Jonckx B. Release of experimental retinal vein occlusions by direct intraluminal injection of ocriplasmin. Br J Ophthalmol. 2016 Dec;100(12):1742-1746. doi: 10.1136/bjophthalmol-2016-309190. Epub 2016 Sep 28. PMID: 27688592; PMCID: PMC5256413.
Gijbels A, Vander Poorten EB, Gorissen B, Devreker A, Stalmans P, Reynaerts D. Experimental validation of a robotic comanipulation and telemanipulation system for retinal surgery. In: Proceedings of the IEEE RAS and EMBS International Conference on Biomedical Robotics and Biomechatronics. ; 2014. doi:10.1109/biorob.2014.6913767
Willekens K, Gijbels A, Schoevaerdts L, Esteveny L, Janssens T, Jonckx B, Feyen JH, Meers C, Reynaerts D, Vander Poorten E, Stalmans P. Robot-assisted retinal vein cannulation in an in vivo porcine retinal vein occlusion model. Acta Ophthalmol. 2017 May;95(3):270-275. doi: 10.1111/aos.13358. Epub 2017 Jan 13. PMID: 28084059.
Figure 2.6.2
The “steady hand of John Hopkins University” is a cooperatively controlled microsurgical system where the surgeon and the actively controlled robotic arm move the surgical instrument simultaneously.
(from Molaei A, Abedloo E, De Smet M, et al. Toward the art of robotic-assisted vitreoretinal surgery. J Ophthalmic Vis Res. 2017.)
Tele‑manipulation systems, are based on the so-called “master-slave” design. The surgeon controls at a distance, a robotic manipulator to which instruments can be attached and used to perform surgical maneuvers and tasks. The surgeon interacts with the manipulator through a motion controller. The controller can be operated by the surgeon either positioned adjacent to the surgical site or at a console (decoupling the surgeon from the patient and the surgical field). Depending on the level of precision needed (controlling ocular and/or patient head movements), chandelier intraocular illumination and/or a dedicated head stabilization or head positioning device may also be required. Motions generated by the controller are fed to a computer that translates and adapts the movement to the robotic arm as a function of the required (intraocular) task. Robotic functionalities such as standby, stored position and advancement bounds are benefits of tele‑operation systems which allow the surgeon to maintain a precise overview and control of movement within the surgical field. Surgical visualization (using current imaging systems) is unchanged, using the oculars of a microscope or digital 3D on an external monitor (Figure 2.6.3).
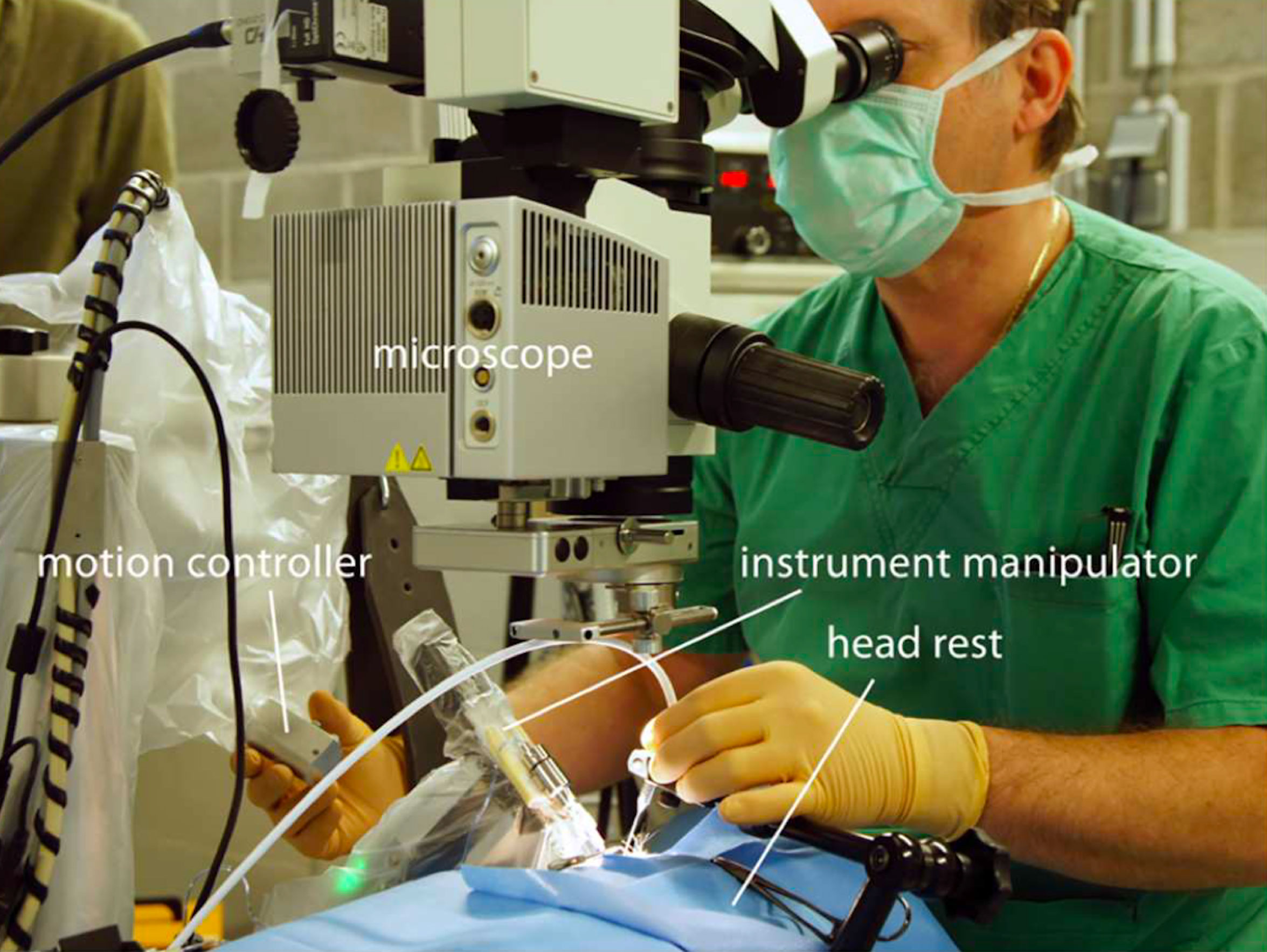
Figure 2.6.3
The Preceyes micromanipulator is positioned for this intervention for the right eye of a pig. The motion controller is handled by the surgeon, from the temporal side of the eye within the surgical field and well within reach of the eye itself. The surgeon remains in his standard operating room position. In his left hand, he is holding a handheld endoilluminator (free hand).
(Image courtesy of PRECEYES b.v. Eindhoven, the Netherlands)
The Preceyes Robotic Surgical System (PRSS) is a telemetric system that has received CE mark approval for use in the vitreo-retinal surgical environment without any limitation to a specific task. Several different intraocular instruments can be fitted to the PSS and are approved for use, increasing the versatility of the platform. Interaction with iOCT and, in the future, OCT probes that are integrated into the tip of instruments will further advance high precision imaging and permit instrument movement boundaries in real time.
To treat certain retinal vascular pathologies such as retinal vein occlusion, arterio-venous malformations or even retinal macroaneurysms, a reliable means of cannulating the retinal vasculature is needed. Unassisted cannulation of retinal veins is possible in cadaver eyes, several disc diameters from the optic nerve.[15,16,17,18] However, at some distance from the optic nerve, assistive devices are required to stabilize the vein or the cannula (in a live setting, as soon as the first branch point in the vasculature is reached, an assistive device is needed). To overcome some of these limitations, robotics has been proposed as a solution. Robotic assistance increases surgical accuracy and precision 5- to 10-fold, while providing tremor damping and motion scaling. Ueta et al. successfully cannulated retinal veins in live cats in a laboratory setting.[19] De Smet et al[11] described a reliable method of retinal vein cannulation by robotic (micromanipulator) assistance that can be carried out using existing surgical equipment and instrumentation (Figure 2.6.4).
Previous
2.5 Intraoperative OCT
All rights reserved. No part of this publication which includes all images and diagrams may be reproduced, distributed, or transmitted in any form or by any means, including photocopying, recording, or other electronic or mechanical methods, without the prior written permission of the authors, except in the case of brief quotations embodied in critical reviews and certain other noncommercial uses permitted by copyright law.
Westmead Eye Manual
This invaluable open-source textbook for eye care professionals summarises the steps ophthalmologists need to perform when examining a patient.